AI in healthcare is a particularly sensitive topic, not least because of personal privacy and the risks involved – it can literally be a matter of life and death. There are plenty of challenges to its uptake that need to be addressed, and one of our Interviews highlighted the key areas these fall into.
First, AI relies on strong data, which means obtaining large, clean and unbiased datasets. Another topic is public and clinical acceptance and trust; without trusting that what is happening to their information is secure, patients won’t want to be involved. And patient safety is paramount, so AI applications need to be trusted to work. Moreover, regulations need to be relevant, but the “regulatory environment is so far behind where we are today with technology”. Finally, there is the issue of proving that AI has real-world, long-term benefits. It is difficult to measure outcomes beyond the immediate and short term in healthcare, but that’s exactly what is needed: “For things like long-term chronic illnesses, how is it that we’re measuring the outcomes of the care… So, if we don’t even know the questions to ask it’s very difficult for AI to come in and be beneficial.”
Some of these are chicken and egg issues; finding the data to show that AI works involves persuading people or governments to use it, but you need data in the first place to prove its worth. Despite this, AI is being used in “highly advanced” areas such as “robotic surgery, in orthopaedics, in script dosage and organ transplant patients”. However, a few more specific areas are seeing wider uptake, “like image analysis and radiology, patient movements or patient tracking in beds, self-care and diagnosis”.

Symptom checking is another area making ground. Babylon Health is one of the most recognised names in this space. Founded in 2013 and headquartered in London, the company offers a symptom checker and remote consultations. What’s more, the UK’s NHS uses an AI-powered chatbot developed by the company. On top of expanding into Rwanda, Babylon has plans in progress with Saudi Arabia, China and the US, and is valued at over USD 2bn following a funding round in mid-2019. The competition includes Ada, based in Germany, which offers symptom checking; Kry, a Swedish company that has partnered with the country’s health authorities; and American Well, an established American telemedicine provider.
For symptom checking and telemedicine companies, international expansion has varying levels of difficulty. It is easier to move between Western-type countries, as a specialist explains of his own experience: “clinical practice and the treatment of diseases and illnesses [are] largely the same from country to country, so that is very transferable internationally. We had no problem moving from the UK to New Zealand, to Australia, and then into Hong Kong as well.” But, as another expert highlights, moving into a new market can mean developing new algorithms to suit “the local illnesses that might only prevail in certain areas, or nutrition or allergies that are common in some parts of the world and others are not.” In addition, many governments require that patients’ medical data is kept within the country, which can mean installing local servers. However, this is more of a cost driver than a limiting factor.
Regulation often lags technological advances and, accordingly, is another issue affecting symptom checkers. In particular, symptom checking does not constitute an actual clinical diagnosis – it is considered just advice. However, if symptom checkers can be proven to provide accurate diagnoses and regulations change, there is the potential for companies to raise prices. The extent of this would depend on the market: “In countries where the out-of-pocket expenditure is high, where the access to a doctor at short notice is very difficult, then the willingness to pay would be a lot higher.”
AI is also assisting R&D efforts, including developing medicine – particularly chemical-based drugs. Biologics such as antibodies are more difficult to design, as “large molecules have a more complicated structure than small ones.” Moreover, there is less data on large molecules, meaning it’s harder to design AI models. Despite this, the amount of large-molecule drugs being released is increasing, accounting for about 30% of new drugs launched to market.
In terms of chemical drugs, the R&D process, which usually takes about 15 years, can be split into two stages: discovery and testing. AI is predominantly used in the former, which can be further divided into three steps. First, the pathogenesis of the disease needs to be mapped. Then, there is screening and drug design to find a chemical molecule able to reverse the process that induces disease. AI is used least in the last stage, in vitro biological testing, although it is used to “evaluate the solubility, toxicity, metabolism and permeation of chemicals.”
AI could transform the development process: “the real value of AI lies in its potential to become an engine for new drug development.” Data is needed to fuel AI, and more is being generated and collected as companies working in this sector recognise its importance, allowing for the development of more models. Meanwhile, deep learning- or network-based solutions can help find new targets. As pointed out by an expert, “sometimes we fail to find an effective treatment for a disease not because of the failure to identify chemicals that act effectively on an existing target, but the failure to identify the right target.”
There are a number of companies around the world involved in AI R&D. When it comes to target identification, twoXAR and BenevolentAI “use existing data to explore the possibilities of synthesising new drugs”. Companies making AI solutions for compound screening include US firms Atomwise, Insilico Medicine and Numerate, as well as UK-based Exscientia and BenevolentAI, while Accutar Biotechnology in China specialises in medicine discovery.
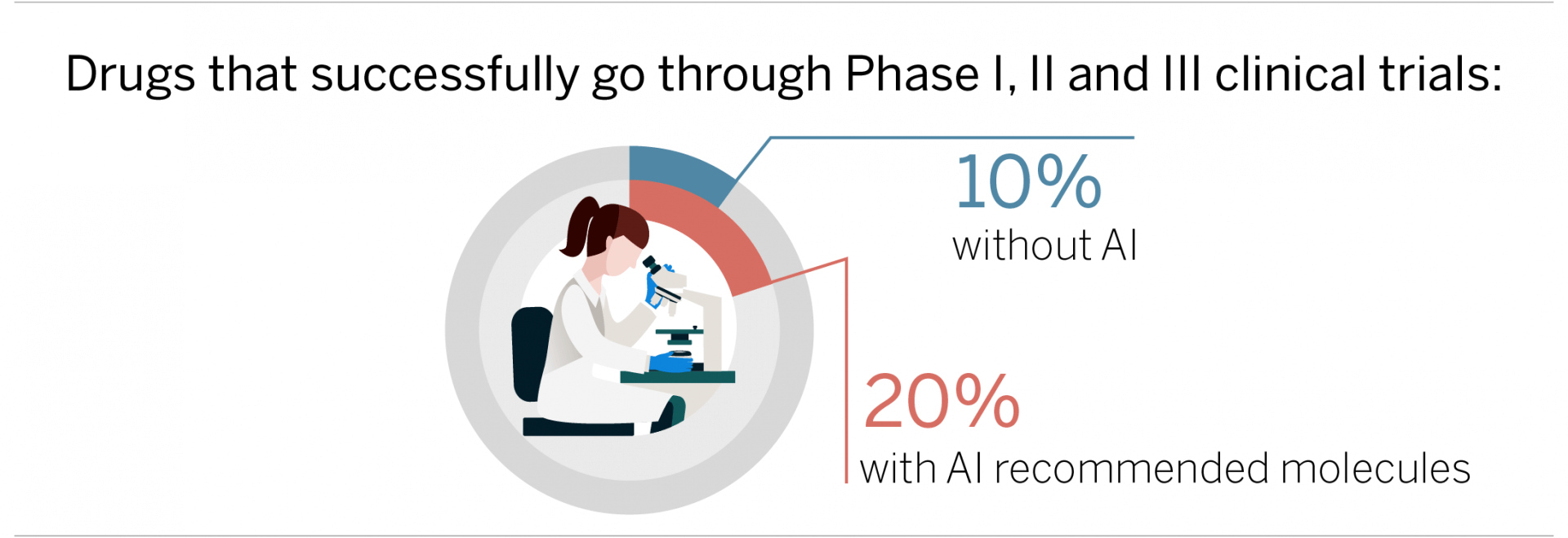
Another area where AI can add value is through recommending more suitable molecules: “The AI-based approach can, in a sense, identify better quality drug candidates at an early stage to ensure a higher success rate in the later stages.” Accordingly, the time spent working on finding molecules is cut – one estimate stands at 30-50% compared with traditional methods. However, the main value lies in delivering better drugs with more chance of making it through the three phases of clinical trials. In fact, our specialist believes that the success rate could be doubled, to 20%.
These examples only touch on how AI is assisting healthcare. Although these are often high-stakes areas, companies have the potential to improve people’s lives. And as technologies such as quantum computing, machine learning and robotics continue to advance, so do the possibilities for how they can transform the industry.
The information used in compiling this document has been obtained by Third Bridge from experts participating in Forum Interviews. Third Bridge does not warrant the accuracy of the information and has not independently verified it. It should not be regarded as a trade recommendation or form the basis of any investment decision.
For any enquiries, please contact sales@thirdbridge.com


