Although still a relatively nascent field, breakthroughs are accelerating, with FDA approvals gaining momentum. The latest are in companion diagnostics (CDx), with FoundationOne Liquid CDx and Guardant360 approved for people with any solid cancer. CDx tests are performed to ascertain what cancer treatment will have the best response in a patient. While the FDA has approved blood tests that detect the presence of a single gene mutation in tumor DNA, these check for multiple cancer-related genetic changes.1https://www.cancer.gov/news-events/cancer-currents-blog/2020/fda-guardant-360-foundation-one-cancer-liquid-biopsy#:~:text=FDA%20approved%20Guardant360%20CDx%20on,that%20have%20been%20proven%20accurate “That was a huge event in the liquid biopsy field, because it was the first time the FDA approved comprehensive tests,” an executive at Boudicca Dx LLC told Third Bridge Forum. The specialist expects more CDx breakthroughs now that Guardant Health Inc and Foundation Medicine, Inc. have laid the path.
We heard there has also been a “burst in monitoring assays” — or minimal residual disease (MRD) tests — where a patient has been treated successfully and is subsequently monitored to swiftly detect potential signs of a return in cancer. Key MRD players are Guardant Health and Natera Inc, both of which focus on colorectal cancer but are working to expand to other types of cancer.
Another major theme is M&A, with a former VP at Exosome Diagnostics Inc referring to “the beginning of a furious market consolidation”, and “high-visibility” start-ups also springing up. Exact Sciences Corp’s acquisition of Thrive Earlier Detection Corp. in October 2020 was described by the former company as a “giant leap toward ensuring blood-based, multi-cancer screening becomes a reality and eventually the standard of care”.2https://investor.exactsciences.com/investor-relations/press-releases/press-release-details/2020/Exact-Sciences-To-Acquire-Thrive-Earlier-Detection-Becoming-A-Leader-In-Blood-Based-Multi-Cancer-Screening/default.aspx The deal was particularly noteworthy because Exact Sciences owns the only FDA-approved stool DNA test for colorectal cancer, Cologuard. The acquisition of Thrive will also expand the firm’s focus in pan-cancer early oncology detection.
“Exact is clearly trying to own the early cancer detection screening space and they’re using their economic power to buy rather than make,” an executive at Natera Inc told Forum. “Whether that strategy will work, it depends… on one, how good their acquisitions are and how good they are at integrating them into an end-to-end solution. It’s a different strategy than one that’s based on placing next-gen sequencing instruments into laboratories, which is what Grail and Illumina are doing.”
Illumina’s acquisition of Grail, announced in September 2020, was seen as an opportunity by the former company to “move into a very, very rapidly growing space, and ultimately build out some of the work that they’re doing potentially down the line with pharma partners or even surrounding extended indications for sequencing”, a former VP at Guardant Health Inc said. Grail was founded by Illumina, which produces the machinery for diagnostic players to conduct tests, in 2016 and spun out as a standalone company. “The advantage of being reunited with Illumina is that Illumina has a very strong position in the genomic tool space. Virtually every laboratory that does next-gen sequencing has some tie to Illumina,” the Natera executive added. However, it is worth noting that the Federal Trade Commission has filed an administrative complaint and authorised a federal court lawsuit to block Illumina’s proposed acquisition of Grail, claiming it would diminish US innovation multi-cancer early detection tests.3https://www.ftc.gov/enforcement/cases-proceedings/201-0144/illumina-inc-grail-inc-matter Forum will continue to monitor this development.
Meanwhile, Grail is gearing up to launch its Galleri multi-cancer screening test commercially this quarter, which would be a huge breakthrough. The company would essentially “become a predicate, so a model that everyone else would follow if they want to develop a test, so that will be huge, whatever is in that label”, the specialist added. However, as noted by the Natera executive, “there have been a lot of false starts in oncology early detection processes”.
The main difference between Illumina and Exact Sciences, the Boudicca Dx executive said, is that Exact Sciences’ products are single-site in vitro diagnostics (IVD) whereas Illumina has a distributed approach. “What that means is Illumina can help Grail make a kit that can be sent to any lab in the world… that’s different from Exact Sciences, which are a single-site IVD. They’re similar to Foundation Medicine and Guardant, where you have to send your samples to an Exact Sciences lab.” The specialist believes the distributed model will be critical for early cancer detection.
Another prominent player in the space is the above-mentioned Guardant Health, which a former VP of the company believes “has the edge” in precision oncology and solid tumours. In addition, rapid test results and having a low cost of goods come with “doing a lot of sequencing and having a well-oiled machine,” they said. “Guardant has got the market share right now and they’ve got the experience and the publications and loyalty among the clinicians to maintain that leadership position, in my opinion”. However, the former VP at Exosome Diagnostics Inc has a more conservative view: “I think Guardant will always be a strong competitor, I just don’t ever think they’re going to be a standout. I say that for a couple of reasons. There’s nothing terribly novel about their technology.”
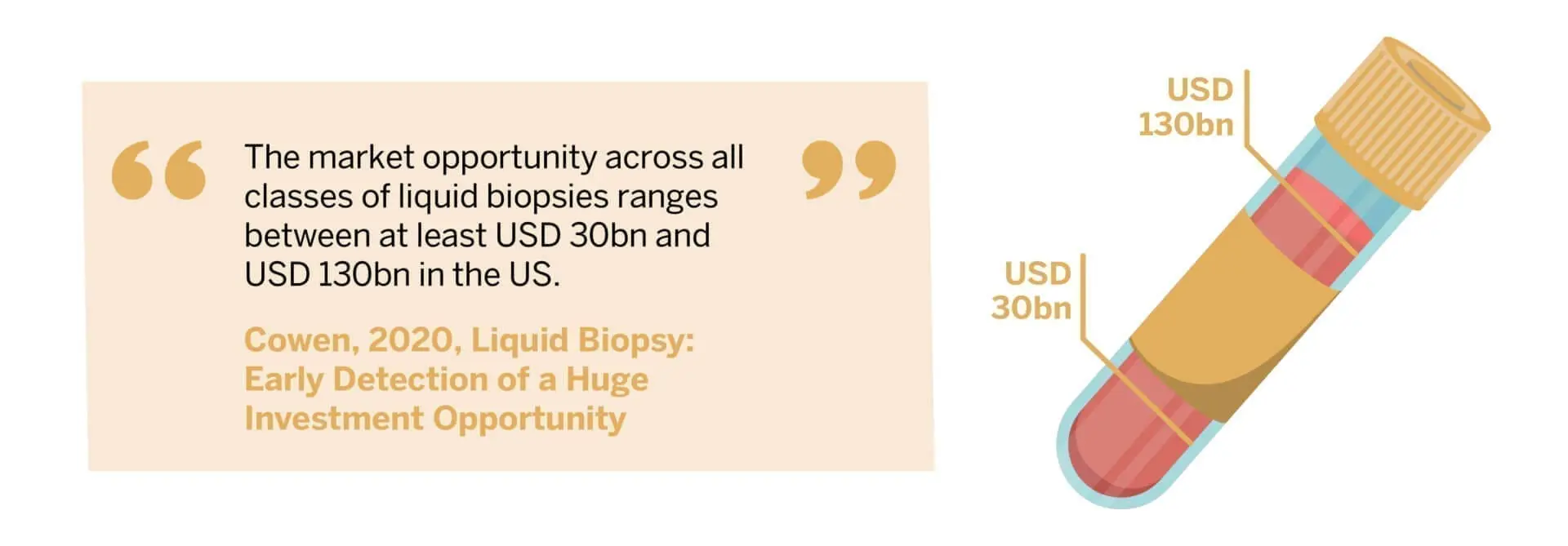
The liquid biopsy market is generating significant interest and investment. According to Cowen, the market opportunity across all classes of liquid biopsies ranges between at least USD 30bn and USD 130bn in the US.4https://www.cowen.com/insights/liquid-biopsy-early-detection-of-a-huge-investment-opportunity/ However, there is a long way to go before commercialisation really takes off and there are still many unknowns and important considerations. For example, how frequent should testing be in early cancer detection and how expensive might such a test be? There are also question marks around how many false positives or negatives are acceptable in a given scenario. Moreover, what exactly happens once these tests are performed, such as how to interpret them for treatment planning, has yet to be determined. This will also be the biggest challenge for reimbursement coverage.
For the time being at least, tissue biopsies are set to retain their “gold standard” status, with Interviews suggesting they will continue to play a vital role in cancer detection and treatment planning. “Sometimes, from one of the first studies we published, you’ll find 80% what you find from blood is picked up by tissue, and 80% vice versa, but there’s still a little bit of a gap, because of heterogeneity, because of tumour shedding, etc,” the former Guardant VP said. Indeed, “while we are getting more and more information which resembles the information we get from microscopy and molecular diagnostics applied to tissue, they’re not 100% overlapping,” the Natera executive said.
Many of the learnings and breakthroughs are also expected to be applicable in other areas, such as organ failure. “There are big markets in adult kidney disease, 40 million Americans have chronic kidney failure, we need better monitoring of that and that’ll impact the dialysis market and the transplant market,” the Natera executive said. Ultimately, “we’re in a world where blood and urine are going to become… much more quantitative and reliable monitors of disease.”
The information used in compiling this document has been obtained by Third Bridge from experts participating in Forum Interviews. Third Bridge does not warrant the accuracy of the information and has not independently verified it. It should not be regarded as a trade recommendation or form the basis of any investment decision.
For any enquiries, please contact sales@thirdbridge.com